Biomaterials, including hydrogels, must exhibit specific mechanical properties to ensure compatibility, functionality, and longevity. Strength, stiffness, density, composition, and viscoelasticity properties influence how biomaterials interact with biological systems.
Therefore, micromechanical measurements are essential to engineer and control the desired biological behaviors of biomaterials and create 3D structures that closely mimic native tissues under physiological conditions.
Biomaterial stiffness must be tuned to match the mechanical properties of natural tissues, ensuring optimal cell function, integration, and stability for applications in biomedical science.
Monitoring viscoelasticity allows biomaterials to mimic the behavior of natural tissues, promoting their functionality, compatibility, and stability.
Mechanical signals from the dynamic microenvironment regulate cell behaviors, especially cell adhesion, influencing how biomaterials interact with biological systems.
Stress relaxation characterizes how biomaterials reduce stress under constant strain over time. Measuring stress relaxation provides insights into the time-dependent mechanical properties of biomaterials and how they respond to mechanical loads.
Creep describes the gradual deformation of biomaterials under a constant load. Evaluating creep behavior in biomaterials helps understand the long-term mechanical changes associated with their performance and durability in various applications.
Incorporating mechanical measurements will:
Measure mechanical properties at the cellular scale
Ensure reliability and reproducibility
Integrate and streamline mechanics within your existing biological workflows
We enable researchers to get the most out of their time and efforts by providing solutions that meet their diverse and versatile needs.
Powerful insights in a small package. Discover our compact, standalone Piuma platform.

Combine unique mechanical insights with the imaging equipment of your choice. Our Chiaro platform is the perfect collaborator.

Discover high-throughput mechanical screening that seamlessly integrates with existing biological workflows, effortless correlation, offering high resolution and reproducibility.

Neves et al. demonstrated that cells embedded in alginate hydrogels can detect spatial cues and sense the 3D topography, simulating the native ECM. They also discovered that in-gel microstructures significantly affect the mechanical heterogeneity of the hydrogels, which is critical for cell delivery applications.
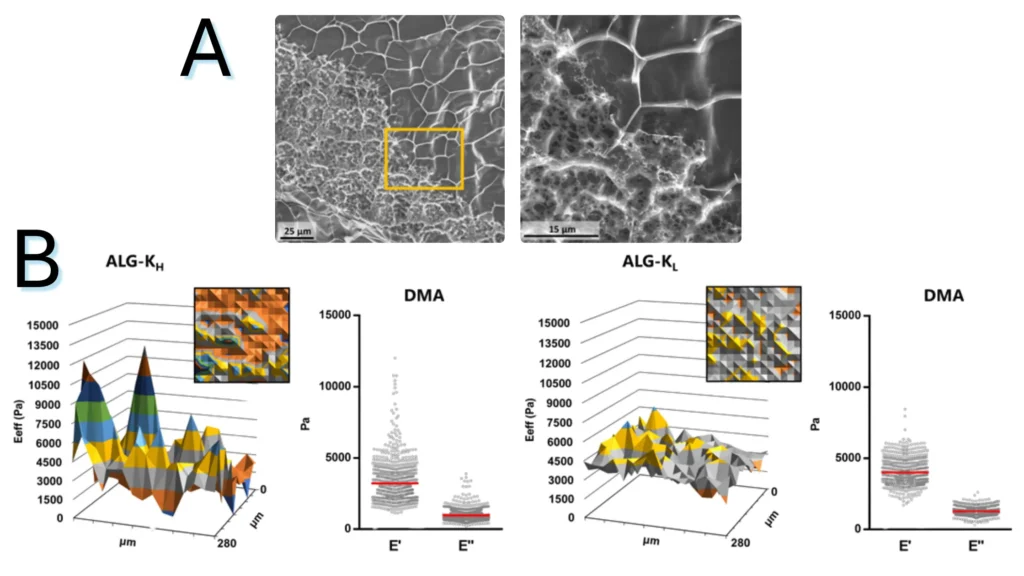
Figure 1.1. Characterization of alginate hydrogel’s structural and mechanical heterogeneity (A) CryoSEM of ALG-KH hydrogels, showing distinct denser/loser regions. (B) Surface mechanical properties of ALG-KH and ALG-KL hydrogels obtained by static (effective Young’s modulus, Eeff) and DMA (elastic, E′, and viscous, E″, components) nanoindentation measurements.
Note. Adapted from “Microstructured click hydrogels for cell contact guidance in 3D”, Neves, MI, 2023, Mater Today Bio. 2023; 19:100604. doi:10.1016/j.mtbio.2023.100604. © 2023 The Authors. Published by Elsevier Ltd. This is an open access article under the CC BY license.
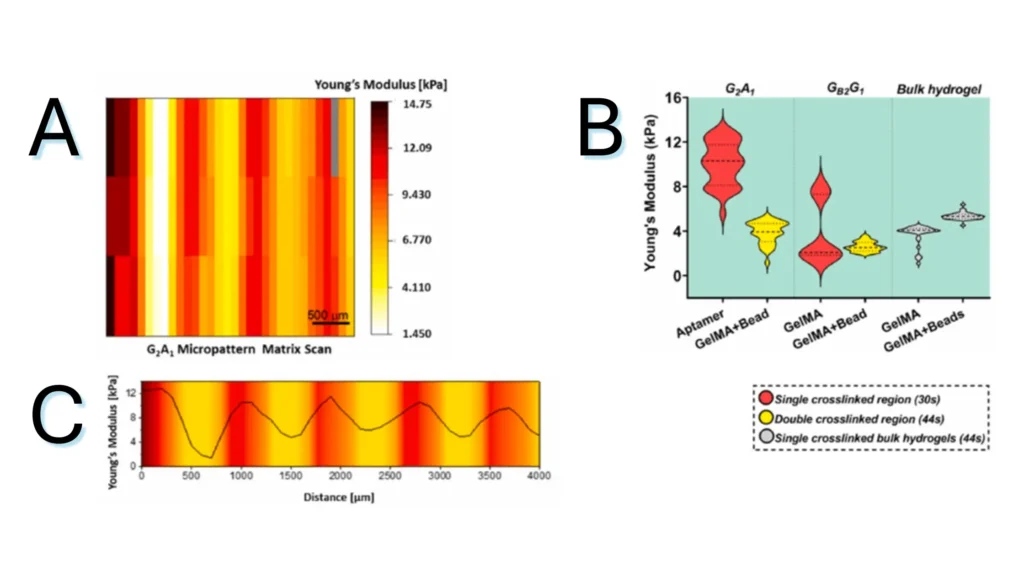
Figure 1.2. Micromechanical behavior of bicomponent micropatterns in hydrogel scaffold. (A) Matrix scan of Young’s modulus of the crosslinked micropattern, revealing the mechanical difference between the aptamer and GelMA regions. (B) Young’s modulus quantification of different regions in photocrosslinked aptamer/GelMA micropatterns or bulk GelMA/GelMA mixed with fluorescent microbeads, revealing the effect of aptamer content on the extent of crosslinking. (C) Young’s modulus profile across the long axis of a micropattern depicting the gradual variation in Young’s modulus magnitude between regions.
Note. Adapted from “Spatial control of self-organizing vascular networks with programmable aptamer-tethered growth factor photopatterning”, Rana, D, 2023, Mater Today Bio, 19:100551. doi: 10.1016/j.mtbio.2023.100551.24. . © 2023 The Authors. This is an open access article under the CC BY license.
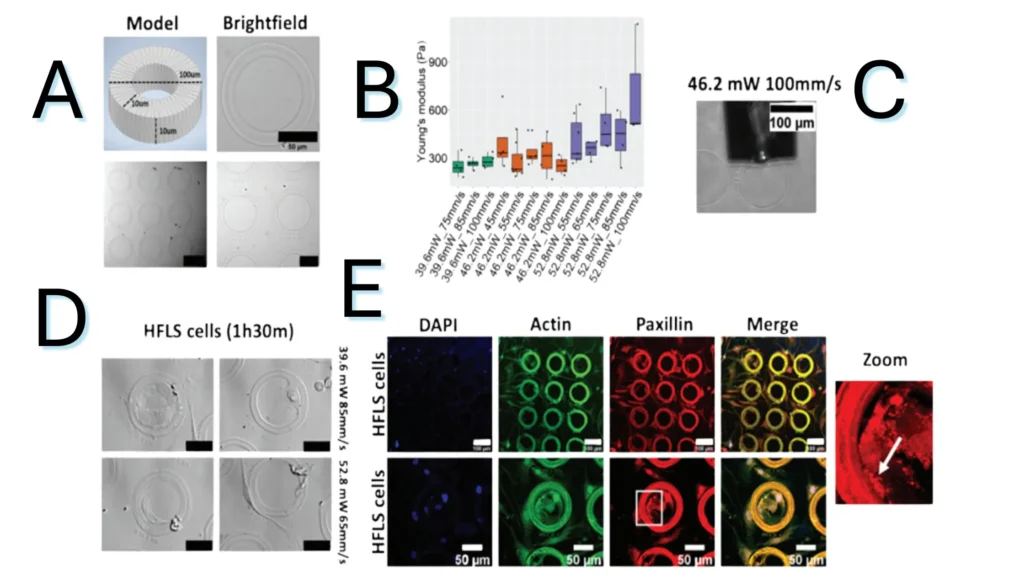
Figure 1.3. Characterization of the hydrogel ink. (A) Model of the hollow pillar and brightfield images of them after the print. (B) Boxplot of the Young’s Modulus (Pa) of different cylinders printed with HYDROBIO INX N100 ink with increasing laser power and speed. (C) Representative image acquired during the analysis with the nanoindenter. (D) Brightfield images of the printed cylinders with cells. (E) Fluorescence analysis on the cells incubated with the hydrogel cylinders.
Note. Adapted from “Two-Photon Laser Printing to Mechanically Stimulate Multicellular Systems in 3D”, Colombo, F, 2024, Adv. Funct. Mater., 34, 2303601. doi: 10.1002/adfm.202303601. © 2024 The Authors. This is an open access article under the CC BY license.






Explore high-throughput mechanical characterization of biomaterials and hydrogels using our indentation platforms.

Monitoring batch-to-batch variability of hydrogel rich in extracellular matrix proteins (Matrigel) to simulate in vivo cell environments.
Whether your focus lies on mechanical measurements and characterization at the cell scale, or you work with muscle tissues, our platforms offer you precise, fast, and accurate outcomes. Discover more about how our products can help you accelerate and achieve your research goals.
With a wide range of application areas, across an array of samples, for various disease areas and testing needs, we can provide you with precise insights into mechanical cues to advance your translational research. Learn more about our applications here.
We are a growing team of 60+ passionate people, headquartered in Amsterdam, the Netherlands. Learn more about our journey so far, meet our team of professionals, and our career opportunities.
Resources
Contact