Limitations of existing experimental models, evaluation methods, and biomarkers result in difficulties replicating the mechanical microenvironment of fibrotic tissues, leading to inconclusive preclinical outcomes.
To overcome these obstacles, mechanical measurements are becoming increasingly relevant in disease modeling and drug development. Efforts focused on quantifying microscale variations in stiffness and viscoelasticity—key mechanical biomarkers of fibrosis progression—pave the way for more accurate and effective research outcomes.
Stiffness is an indicator of fibrosis stages, as matrix stiffness increases during tissue fibrosis. Measuring stiffness helps evaluate disease progression and potential antifibrotic therapies.
Loss of tissue viscoelasticity impairs organ functionality in fibrosis. Understanding the viscoelastic behavior of tissues can provide insights into disease mechanisms and support treatments to restore tissue mechanics.
Cell adhesions maintain tissue integrity and act as mechanotransducers by converting mechanical cues into biochemical signals, with alterations in these adhesions linked to fibrotic conditions. Measuring cell adhesion in fibrotic models can reveal how altered mechanical signals drive the disease and test interventions to normalize cell interactions.
Stress relaxation characterizes how tissues reduce stress under constant strain over time. Measuring stress relaxation provides insights into the time-dependent mechanical properties of fibrotic tissues and how they respond to mechanical loads.
Creep describes the gradual deformation of tissues under a constant load. Evaluating creep behavior in fibrotic tissues helps understand the long-term mechanical changes associated with disease progression and treatment effects.
Incorporating mechanical measurements will:
Enable more physiologically relevant experimental outcomes
Improve the selection process for lead drug candidates in the preclinical phase
Accelerate drug development timelines
Reduce the risk of clinical trial failures by improving early-phase testing and validation
Integrate and streamline mechanics within your existing biological workflows
Minimize setbacks that could lead to significant losses and project delays
We enable researchers to get the most out of their time and efforts by providing solutions that meet their diverse and versatile needs.
Powerful insights in a small package. Discover our compact, standalone Piuma platform.

Combine unique mechanical insights with the imaging equipment of your choice. Our Chiaro platform is the perfect collaborator.

Discover high-throughput mechanical screening that seamlessly integrates with existing biological workflows, effortless correlation, offering high resolution and reproducibility.

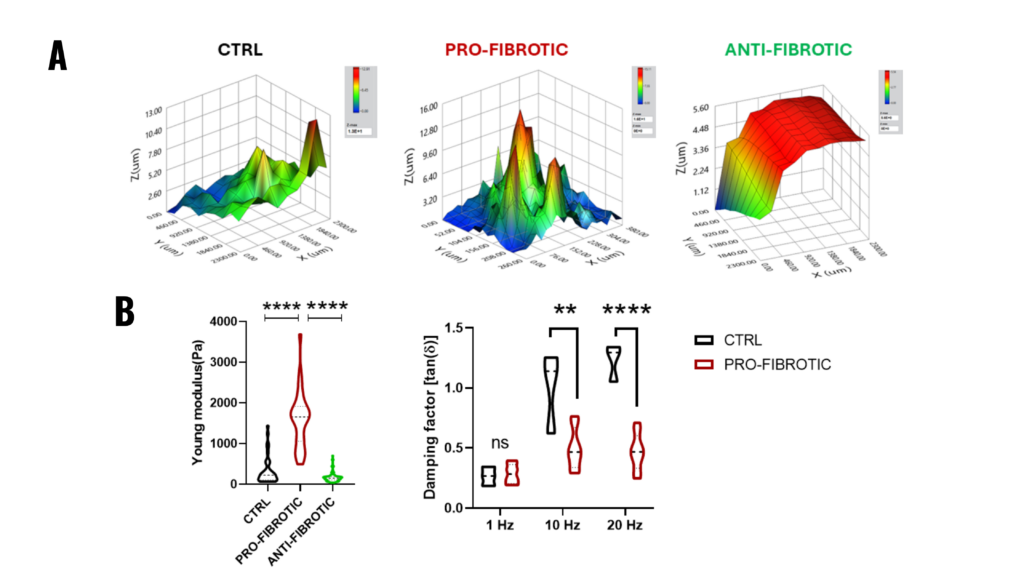
Figure 2.1. Mechanical properties of fibrotic and non-fibrotic dECM measured by Pavone. (A) Topographic maps of the dECM’s surface. (B) (Left) Violin plot representation of Young’s modulus analysis of the dECMs. (Right) Dynamic measurements of viscoelastic modulus indented at a frequency of 1-10-20 Hz expressed as the viscous to elastic properties ratio of the material (tan, δ).
Note. Adapted from “Fibrotic extracellular matrix impacts cardiomyocyte phenotype and function in an iPSC-derived isogenic model of cardiac fibrosis”, Niro, F, 2024, Translational Research. 2024; 273;58-77. doi: 10.1016/j.trsl.2024.07.003. 1931-5244/©2024 The Authors. Published by Elsevier Inc. This is an open access article under the CC BY license.
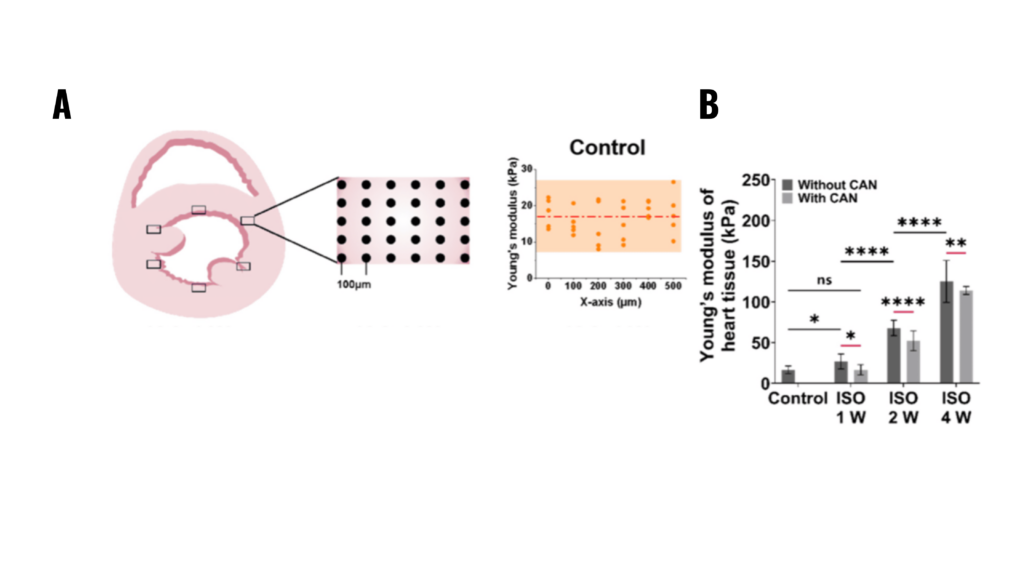
Figure 2.2. Characterization results of myocardial tissue stiffness and collagen deposition in myocardial fibrosis rats without and with CAN treatment. (A) Stiffness diagrams of tissue samples were obtained based on their Young’s moduli measured by nanoindentation instrument. (B) Statistical histograms of tissue stiffness of fibrotic myocardium (n = 5).
Note. Adapted from “Effect of Extracellular Matrix Stiffness on Candesartan Efficacy in Anti-Fibrosis and Antioxidation”, Zhu, T, 2023, Oxidative Stress in Cardiac Disease. 2023; 12(3), 679. doi: 10.3390/antiox12030679. © 2023 by the authors. Licensee MDPI, Basel, Switzerland. This is an open access article under the CC BY license.
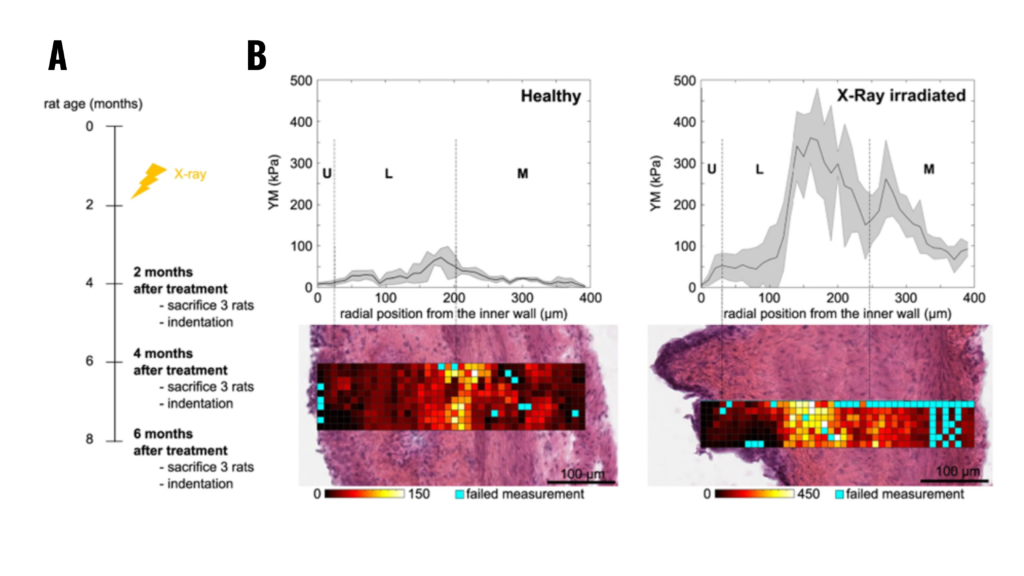
Figure 2.3. (A) Schematic representation of the experiment: X-ray radiotherapy is used to induce actinic cystitis on the bladder (B) Representative bladder wall stiffness gradient collected 4 months after treatment: X-rays cause a stiffening of the whole bladder wall compared to non-treated healthy animals. Mechanical spatial differences within the fibrotic bladder are maintained and associated to the different tissue layers (U: urothelium, L: lamina propria, M: muscle).
Note. Adapted from “Micro-mechanical fingerprints of the rat bladder change in actinic cystitis and tumor presence”, Martinez-Vidal, L., 2023, Commun Biol., 6(1):217. doi: 10.1038/s42003-023-04572-0.19. © The Author(s) 2023. This is an open access article under the CC BY license.






Explore high-throughput mechanical characterization of fibrotic tissues using the Pavone Nanoindenter.

Using micro-mechanics and transcriptional profiling to compare cell biology of patient fibrotic tissues.

Probe how fibrosis reshapes cardiac dECM mechanics to unveil novel therapeutic avenues.
Whether your focus lies on mechanical measurements and characterization at the cell scale, or you work with muscle tissues, our platforms offer you precise, fast, and accurate outcomes. Discover more about how our products can help you accelerate and achieve your research goals.
With a wide range of application areas, across an array of samples, for various disease areas and testing needs, we can provide you with precise insights into mechanical cues to advance your translational research. Learn more about our applications here.
We are a growing team of 60+ passionate people, headquartered in Amsterdam, the Netherlands. Learn more about our journey so far, meet our team of professionals, and our career opportunities.
Resources
Contact