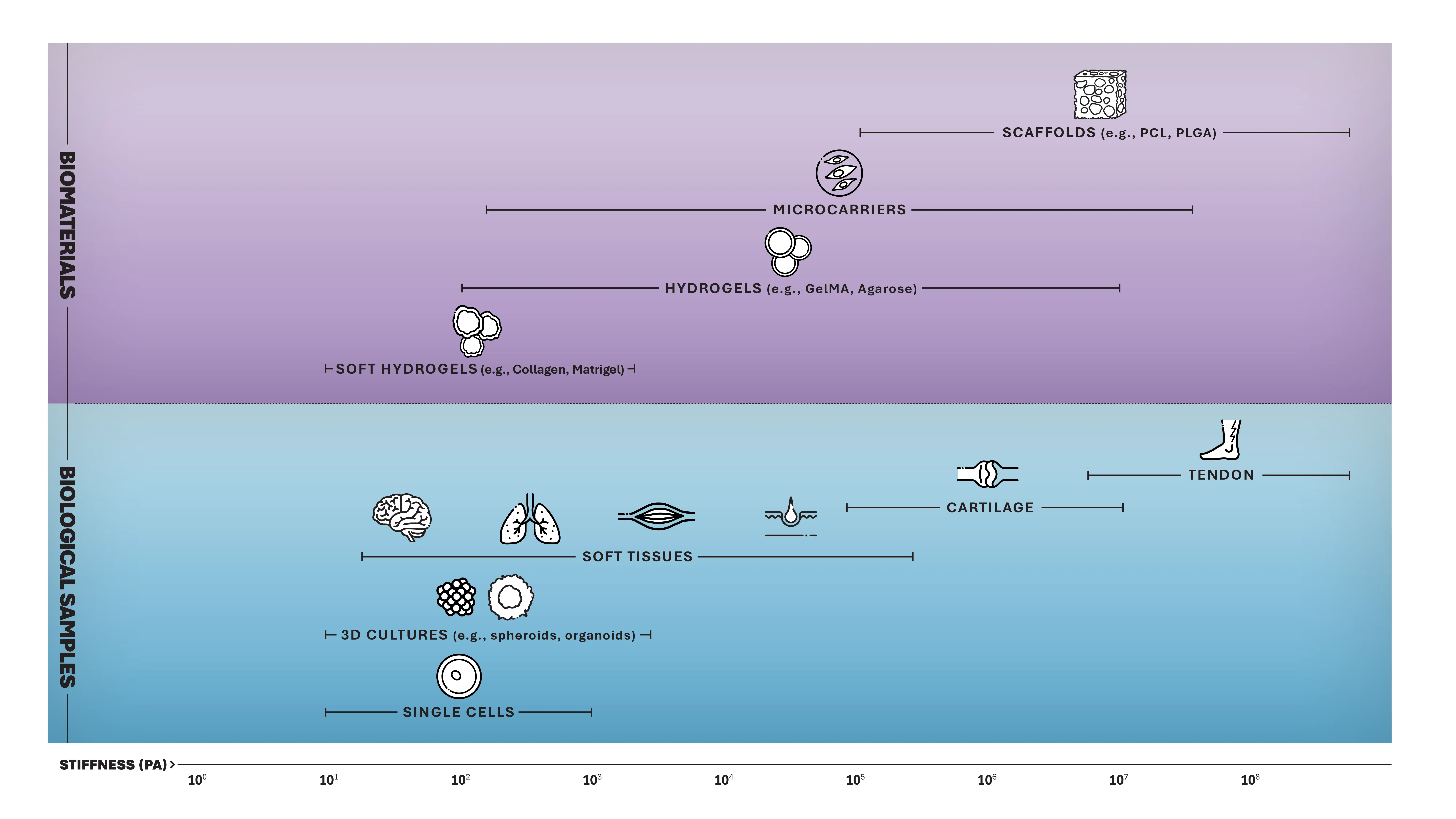
Bridge the gap between biology, physics, engineering, and materials science
Mechanobiology explores how physical forces and mechanical properties shape living organisms’ structure, function, and behavior, from single cells to complex tissues. It delves into how cells sense (mechanosensation) and respond (mechanotransduction) to mechanical stimuli. Variations in mechanical properties, including stiffness and viscoelasticity, provide critical insights into health or disease, serving as unique, label-free biomarkers for diseases and potential treatments in preclinical settings.
Mechanobiology also extends to the study of how mechanical forces interface with biomaterials. These materials are designed not only to provide structural support but also to actively engage with the surrounding biological environment. By manipulating mechanical cues, scientists can engineer biomaterials to regulate specific biological responses and create 3D structures that closely replicate native tissues.
Stiffness is an indicator of the disease’s pathophysiology. Measuring stiffness helps evaluate disease progression and potential therapies.
Loss of tissue (visco)elasticity impairs organ functionality. Understanding the (visco)elastic behavior of tissues can provide insights into disease mechanisms and support treatments to restore tissue mechanics.
Cell adhesions maintain tissue integrity and act as mechanotransducers by converting mechanical cues into biochemical signals, with alterations in these adhesions linked to diseases. Measuring cell adhesion in preclinical models can reveal how altered mechanical signals drive the disease and test interventions to normalize cell interactions.
Creep describes the gradual deformation of tissues under a constant load. Evaluating creep behavior in tissues helps understand the long-term mechanical changes associated with disease progression and treatment effects.
Mechanobiology quantifies the hidden forces that traditional assays (such as cell viability, proliferation, migration, and differentiation) might overlook. By integrating automated mechanical readouts, our easy-to-use fiber optic interferometry-based indentation platforms enhance existing pre-clinical research workflow with a new data dimension.
Enhance experimental outcomes
Enable more physiologically relevant experimental outcomes;
Correlate mechanical data with chemical and biological experimental methods
Ensure reliability and reproducibility of in vitro models, ex vivo systems, and biomaterials;
Control and monitor in vitro microenvironment under physiological conditions
Measure mechanical properties at the cellular scale
Advance early-phase testing and validation of potential therapies and medical devices.
Integrate and streamline mechanics within your existing biological workflows
We enable researchers to get the most out of their time and efforts by providing solutions that meet their diverse and versatile needs.
Powerful insights in a small package. Discover our compact, standalone Piuma platform.

Combine unique mechanical insights with the imaging equipment of your choice. Our Chiaro platform is the perfect collaborator.

Discover high-throughput mechanical screening that seamlessly integrates with existing biological workflows, effortless correlation, offering high resolution and reproducibility.

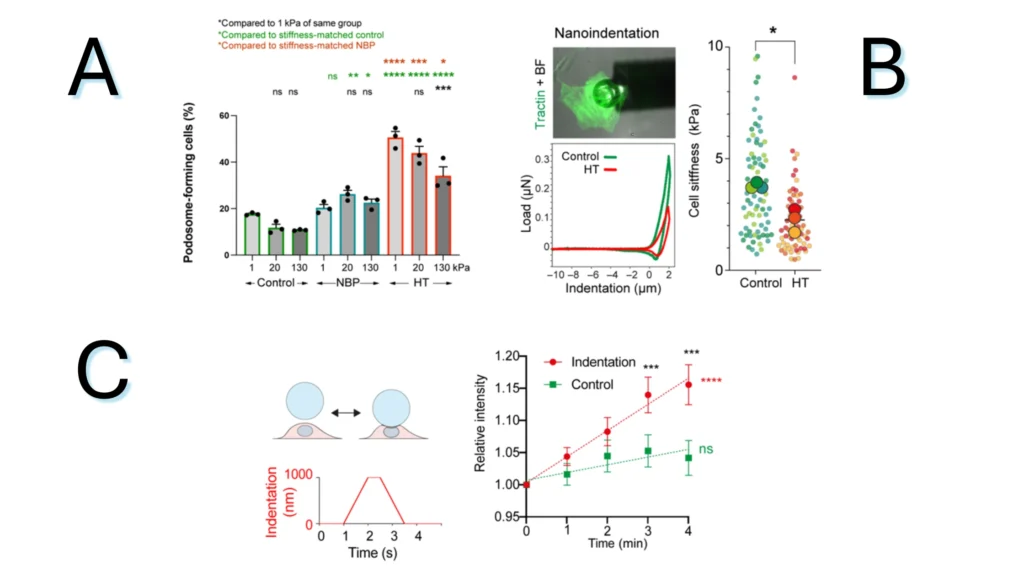
Figure 2.1. The impact of the local mechanical environment on single cells. (A) Percentage of podosome-forming cells in normal blood pressure (NBP) and systolic hypertension (HT) conditions. (B) Cellular stiffness after HT treatment and schematic of single cell indentation. (C) Pressure application with the nanoindenter. Ca2+ levels within VSMCs after a single indentation..
Note. Adapted from Adapted from “Pressure and stiffness sensing together regulate vascular smooth muscle cell phenotype switching”, by Swiatlowska, P.,2022, Sci Adv, 8(15):eabm3471. doi: 10.1126/sciadv.abm3471. ©2024 The Authors. This is an open access article under the CC BY license.
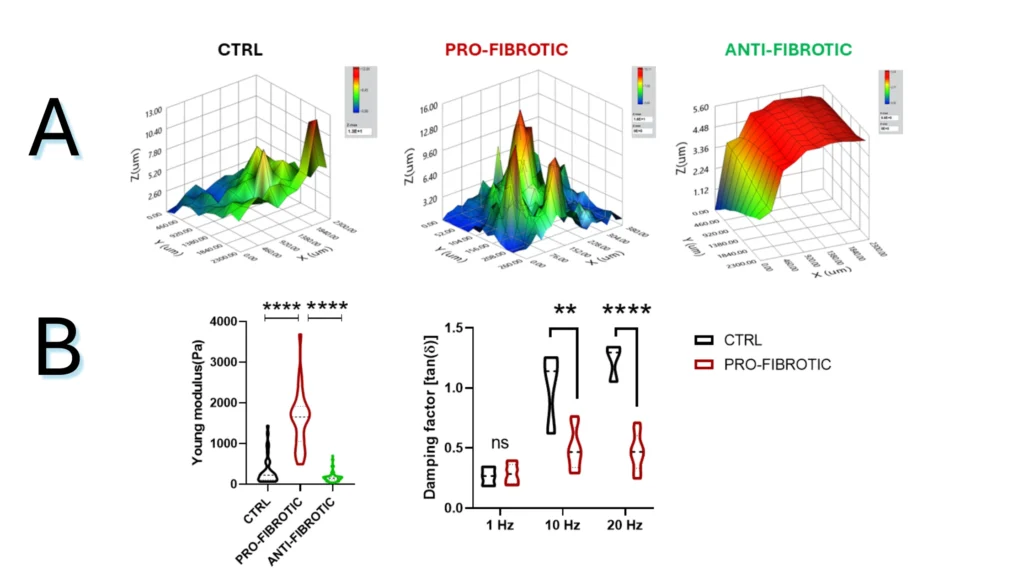
Figure 2.2. Mechanical properties of fibrotic and non-fibrotic dECM measured by Pavone. (A) Topographic maps of the dECM’s surface. (B) Violin plot representation of Young’s modulus analysis of the dECMs. (D) Dynamic measurements of viscoelastic modulus indented at a frequency of 1-10-20 Hz expressed as the viscous to elastic properties ratio of the material (tan, δ).
Note. Adapted from “Fibrotic extracellular matrix impacts cardiomyocyte phenotype and function in an iPSC-derived isogenic model of cardiac fibrosis”, Niro, F, 2024, Translational Research. 2024; 273;58-77. doi: 10.1016/j.trsl.2024.07.003. 1931-5244/©2024 The Authors. Published by Elsevier Inc. This is an open access article under the CC BY license.
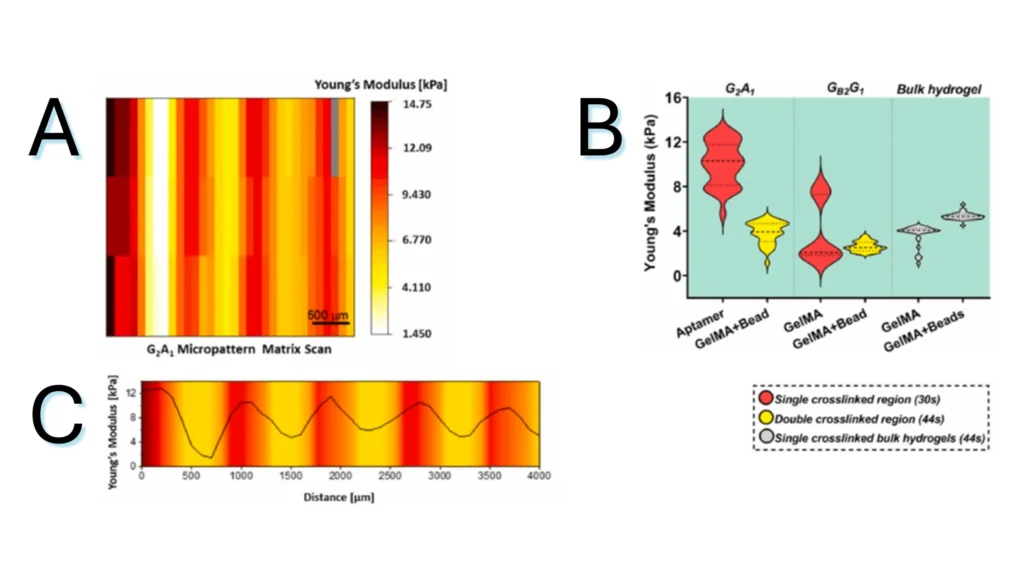
Figure 2.3. Micromechanical behavior of bicomponent micropatterns in hydrogel scaffold. (A) Matrix scan of Young’s modulus of the crosslinked micropattern, revealing the mechanical difference between the aptamer and GelMA regions. (B) Young’s modulus quantification of different regions in photocrosslinked aptamer/GelMA micropatterns or bulk GelMA/GelMA mixed with fluorescent microbeads, revealing the effect of aptamer content on the extent of crosslinking. (C) Young’s modulus profile across the long axis of a micropattern depicting the gradual variation in Young’s modulus magnitude between regions.
Note. Adapted from “Spatial control of self-organizing vascular networks with programmable aptamer-tethered growth factor photopatterning”, Rana, D, 2023, Mater Today Bio, 19:100551. doi: 10.1016/j.mtbio.2023.100551.24. . © 2023 The Authors. This is an open access article under the CC BY license.





Fibrosis disease modeling by tracking mechanical changes at the extracellular matrix microenvironment.

Non-destructive biomaterials testing at physiologically relevant environments.
Why biologists shouldn't be afraid of mechanics. Explore mechanobiology.

Uncover high-resolution mechanical testing across scales with our indentation technology.

Mechanical biomarkers provide essential evidence about the pathophysiology of diseases.

Measuring mechanics in situ within an integrated setup, simplifying the workflow and enhancing data reliability.

Explore high-throughput mechanical characterization of fibrotic tissues using the Pavone Nanoindenter.

Using micro-mechanics and transcriptional profiling to compare cell biology of patient fibrotic tissues.

High-throughput automated hydrogel indentation with the Pavone.
Whether your focus lies on mechanical measurements and characterization at the cell scale, or you work with muscle tissues, our platforms offer you precise, fast, and accurate outcomes. Discover more about how our products can help you accelerate and achieve your research goals.
With a wide range of application areas, across an array of samples, for various disease areas and testing needs, we can provide you with precise insights into mechanical cues to advance your translational research. Learn more about our applications here.
We are a growing team of 60+ passionate people, headquartered in Amsterdam, the Netherlands. Learn more about our journey so far, meet our team of professionals, and our career opportunities.
Resources
Contact