The impact of extracellular matrix stiffness on 3D in vitro models
By Optics11 Life
The extracellular matrix (ECM) is a complex and dynamic network surrounding cells across various tissues and providing structural and mechanical support. Variations in mechanical properties of ECM, such as topography and stiffness, can significantly impact cellular behavior and function. In this sense, different approaches aim to reproduce cell-ECM to recapitulate functional tissues and organs in vitro. This white paper describes the impact of ECM on organoids and the latest advancements in ECM mechanobiology with Optics11 Life technology.
Introduction
The extracellular matrix (ECM) is a complex and dynamic network surrounding cells across various tissues and providing structural and mechanical support. The ECM also mediates numerous biochemical interactions to guide cell survival, proliferation, differentiation, and migration¹,². Simultaneously, the cells actively remodel the ECM, impacting cellular functionalities. This reciprocal interaction between cells and the ECM is central to regulating and controlling morphogenetic and histogenetic processes. Despite being composed of the same structural components—namely elastin, collagen, hyaluronan, proteoglycans, fibronectin, and laminin—the organization and quantity of structural units of ECM differ across various organs³. Therefore, tissue engineering approaches aim to reproduce several biological parameters in vitro, such as cell–cell interaction, cell–matrix interaction, and tissue-specific physiological mechanisms, to recapitulate functional tissues and organs.
This white paper describes the impact of ECM on organoids and the latest advancements in ECM mechanobiology with Optics11 Life technology for customizing 3D in vitro models.
A. Mechanical properties of ECM
The mechanical properties of the ECM can affect cellular behavior and function. Cells attach to the ECM through adhesion receptors and respond to the ECM stiffness by adapting their cytoskeleton. This adaptive response generates a mechanical tension, influencing diverse cell functions such as spreading, motility, apoptosis, signal transduction, and ECM remodeling. Variations in ECM topography, stiffness, and deformability can significantly modify the mechanical tension, impacting cellular processes⁴.
Recent studies have demonstrated the impact of cell–matrix interactions and the mechanical properties of the ECM on three-dimensional (3D) in vitro models, such as organoids⁵–⁸. As organoids become the golden standard for biological research in 3D, ensuring control of their mechanical properties can contribute to generating more reproducible and physiologically relevant in vitro models. Therefore, integrating mechanobiological readouts into organoid research can reveal how mechanical forces contribute to physiological and pathological processes⁹. Moreover, ECM mechanobiology enables the design of scaffolds conducive to organoid development and expansion with defined mechanical properties tuned to specific applications¹⁰.
B. ECM for organoid growth
The growth and maturation of organoids often involve embedding them within ECM-based hydrogels as scaffolds, which present various stiffness, topography, permeability, and degradability levels that influence cellular phenotype, morphology, and function¹⁰. Matrigel© is a widely used hydrogel to generate organoids, derived from a basement membrane matrix secreted by Engelbreth–Holm–Swarm (EHS) mouse sarcoma cells. It comprises ECM proteins, including laminin, collagen IV, heparan sulfate proteoglycans, entactin/nidogen, and several growth factors¹¹,¹². By varying the protein concentration, it becomes softer or stiffer. The higher the polymer concentration, the more elastic (less viscous) the matrix¹³. However, its application in translational therapies is limited due to tumor-derived factors and the challenges of creating specialized organoid niches. Moreover, lot-to-lot variability also limits the clinical application of this biomaterial¹⁴.
Given these limitations, several studies with natural or synthetic-based materials have focused on novel alternatives for organoid cultures¹⁵,¹⁶. Natural polymers derived from ECM offer biocompatibility, biodegradability, and specific signaling cues, but their processability and rheological properties can be limited. Decellularized tissue for acellular ECM and ECM hydrogels are native patient-derived ECM with preserved tissue architecture, yet batch-quality control remains an issue. Conversely, synthetic polymers offer reproducibility and precise control over their mechanical properties and chemical composition. However, they fail to fully replicate native ECM features and complex multifactorial cues for directing cell behavior in organoids¹¹,¹⁴.
ECM’s stiffness characterization using Optics11 Life technology
A. Matrigel©
Ramadan et al.¹⁷ compared the effect of two natural ECM-derived matrices with similar physical properties, the basement membrane (Matrigel) and the interstitial matrix (collagen I), on the intestinal epithelium. Young’s modulus was measured with nanoindentation using a spherical probe with a radius of 50 μm and a cantilever stiffness of 0.5 N/m.
Their findings revealed that collagen type I concentration has a stiffness similar to Matrigel (Figure 1A). They also demonstrated that changing ECM components while maintaining a similar stiffness affects the epithelium via laminin signaling through the ITGA6 receptor (Figure 1B). Blocking ITGA6 diminished the induced morphological changes and prevented the upregulation of intestinal stem cell genes. Overall, Optics11 Life technology allowed the researchers to compare ECM with similar stiffness and conclude that ECM composition influences the behavior of the intestinal epithelium.
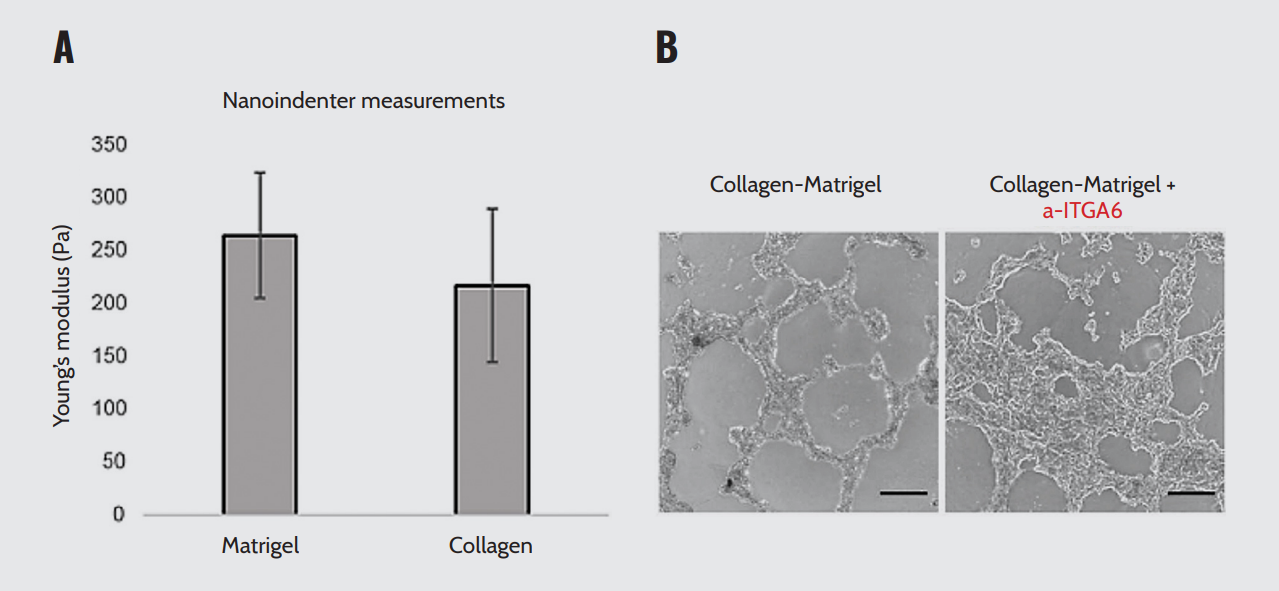
ECM stiffness and laminin concentration. (A) Stiffness of collagen or Matrigel. (B) Inhibition of ITGA6 blocks the downregulation of fetal-like markers and blocks the increase of intestinal stem cell markers. Note. Adapted from “The extracellular matrix controls stem cell specification and crypt morphology in the developing and adult mouse gut”, by Ramadan, R., 2022, Biol Open,
11(12):bio059544. doi: 10.1242/bio.059544.
B. ECM hydrogels
Giobbe et al.⁵ have demonstrated that ECM-derived hydrogels from decellularized tissues exhibit similar physiological and mechanical properties as commercially available gels, such as Matrigel. The hydrogels, derived from decellularized porcine small intestine mucosa/submucosa, support the culture of intestinal organoids and cells from other endoderm-derived tissues, including the liver, stomach, and pancreas (Figure 2A).
The stiffness of the hydrogels was measured using nanoindentation on Petri dishes containing 30 μL ECM gels and Matrigel droplets immersed in phosphate-buffered saline. The probe parameters were a probe stiffness of 0.44 N/m with a tip radius of 57 μm. Consistently, the ECM hydrogels displayed an elastic modulus comparable to Matrigel (Figure 2B). Furthermore, the intestinal ECM-derived hydrogels exhibited a proteomic signature characteristic of endoderm tissue, with specific enrichment of essential ECM proteins for organoid formation. These findings substantiate the presence of a higher fraction of crypt/stem cells in ECM-cultured human intestine organoids (Figure 2C). Optics11 Life technology helped the researchers show that ECM hydrogels derived from decellularized tissues provide a mechanical environment capable of directing the formation and growth of endodermal organoids.

(A) 3D culture of endodermal organoids in ECM gel and Matrigel. Scale bar: 25 μm. (B) Elastic modulus measured by nanoindentation of 6 mg/mL ECM gel vs. Matrigel in 30 μL drops. (C) Real-time PCR analysis of small intestine transcripts.
Note. Adapted from “Extracellular matrix hydrogel derived from decellularized tissues enables endodermal organoid culture”, Giobbe, G.G., 2019, Nat Commun, 10(1):5658. doi: 10.1038/s41467-019-13605-4
Willemse et al.⁶ describe the culture and expansion of human cholangiocyte organoids in liver ECM (LECM)-derived hydrogels. They investigated whether hydrogels derived from healthy porcine LECM (PLECM) or human LECM (HLECM) can replace mouse tumor-derived basement membrane extracts (BME) for the culture and expansion of cholangiocyte organoids.
Nanoindentation experiments revealed no significant differences between the Young’s modulus of PLECM and HLECM (Figure 3A). However, Matrigel showed a significantly higher Young’s modulus than LECM and BME 70%. Moreover, increasing the concentration of the PLECM and HLECM hydrogels (6 mg/ml) did not yield an increased effective Young’s modulus (Figure 3D), indicating less efficient solidification of the LECM hydrogels at higher concentrations. No significant differences were found between PLECM and HLECM at similar concentrations (6.0 mg/ml and 4.5 mg/ml). Additionally, these hydrogels support the proliferation of cholangiocyte organoids and maintain the cholangiocyte-like phenotype. In conclusion, they found that liver ECM hydrogels can successfully replace tumor-derived BME and unlock the full clinical potential of human cholangiocyte organoids. Optics11 Life technology has helped researchers evaluate and select hydrogels with adequate stiffness for cholangiocyte organoid culture and expansion.
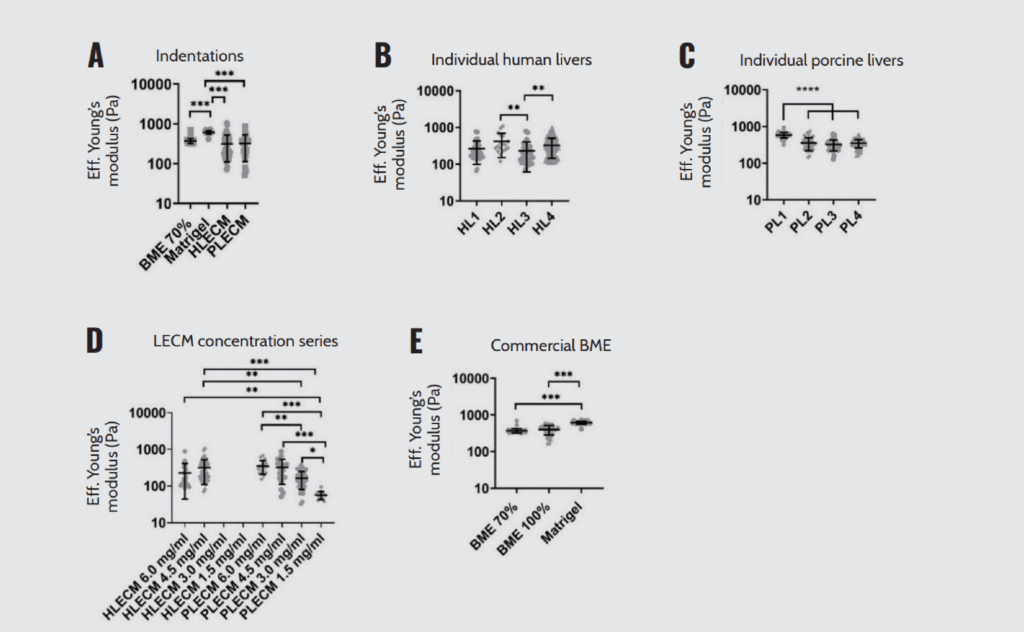
Mechanical characterization of LECM hydrogels by nanoindentation. (A) Young’s modulus of BME 70%, Matrigel, HLECM, and PLECM. (B-C) Young’s modulus of HLECM (B) and PLECM (C) from individual livers. (D) Young’s modulus of LECM and PLECM dilution series (E) Young’s modulus of different BME formulations and Matrigel.
Note. Adapted from “Hydrogels derived from decellularized liver tissue support the growth and differentiation of cholangiocyte organoids”, Willemse, J., 2022, Biomaterials, 284:121473. doi.org/10.1016/j.biomaterials.2022.121473.
C. Tissue-derived ECM
Van Tienderen et al.⁷ created a 3D in vitro model composed of epithelial tumor cells and their surrounding ECM to study metastatic cell–matrix interactions. For that, they combined patient-derived cholangiocarcinoma (CCA) organoids with decellularized human lung (dLu) and decellularized human lymph nodes (dLN). Decellularization resulted in the removal of cells while preserving the ECM structure and retaining significant characteristics of the tissue origin. The decellularized tissues were biochemically and biomechanically characterized, which revealed that decellularized matrix from lung and lymph nodes have a distinct biochemical signature and retain tissue-specific function-related proteins.
They found that macro- and microscale mechanical properties, as determined by rheology and microindentation, revealed local heterogeneity of the ECM. While dLu and dLN have similar stiffness, the macroscale stiffness of dLN has higher heterogeneity (Figure 4A). On a microscale, the stiffness values ranged from 0.15–52.3 kPa for dLu and 0.05–40.9 kPa for dLN (Figure 4B). Effective Young’s modulus split per donor for both dLu and dLN showed no significant differences (Figure 4C).
The heterogeneity in mechanical properties in dLN is mimicked by diversity in ECM proteins, showing the correlation between mechanical and chemical properties of the extracellular environment. These findings indicate that cholangiocarcinoma organoids activate tumor-specific programs in different ECM. This highlights the relevance of choosing a suitable scaffold for organoid culture by mechanically characterizing the ECM. In this context, Optics11 Life technology enabled the researchers to mechanically characterize ECM-decellularized tissues, understand their local heterogeneity, and establish the correlation between their mechanical and chemical properties.
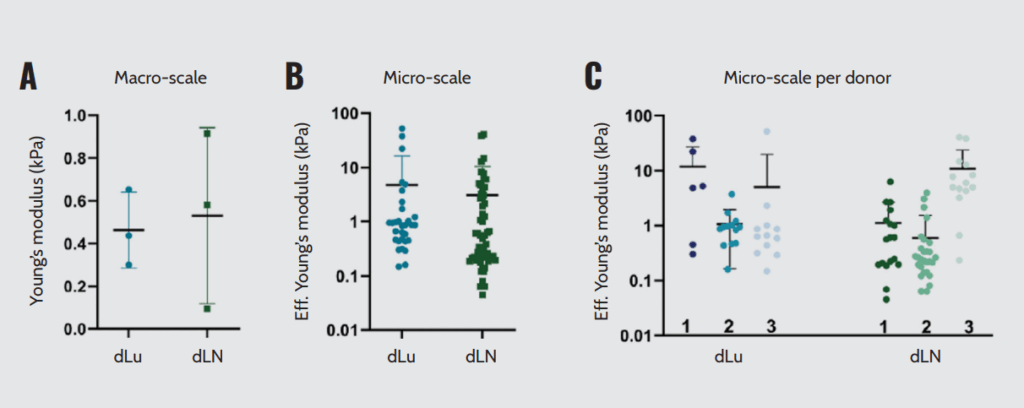
Macroscopic and microscopic mechanical characterization of dLu and dLN. (A) Macroscopic compression measurements showing Young’s Modulus. (B) Effective Young’s Modulus measured by micro-indentation (3×3 matrix scans, 5 μm between indentations with a total measured area of 15×15 μm). (C) Effective Young’s Modulus assessed by micro-indentation split per donor. Each data point is a different region of the sample obtained in a 3×3 matrix scan.
Note. Adapted from “Modelling metastatic colonization of cholangiocarcinoma organoids in decellularized lung and lymph nodes”, van Tienderen G.S., 2023, Front Oncol., 12:1101901. doi: 10.3389/fonc.2022.1101901.
Conclusion
The ECM is not a passive scaffold that statically supports 3D in vitro models, as its biochemical and mechanical composition modulates cellular organization and the subsequent morphology and physiological function of tissues. Therefore, integrating mechanobiology into cell-ECM interactions can create more physiologically relevant in vitro models. Here, we describe the role of ECM in organoid cultures and review how Optics11 Life technology has impacted research groups in understanding ECM mechanics and developing custom 3D models.
Disclaimer
All materials have been reproduced in accordance with the Creative Commons BY (CC-BY) license.
References
- Muiznieks LD, Keeley FW. Molecular assembly and mechanical properties of the extracellular matrix: a fibrous protein perspective. Biochim Biophys Acta. 2013 Jul;1832(7):866–75.
- Hu M, et al. Extracellular matrix dynamics: tracking in biological systems and their implications. J Biol Eng. 2022 May 30;16(1):13.
- Urciuolo F, et al. In vitro strategies for mimicking dynamic cell–ECM reciprocity in 3D culture models. Front Bioeng Biotechnol. 2023 Jun 26;11:1197075.
- Black LD, et al. Mechanical and failure properties of extracellular matrix sheets as a function of structural protein composition. Biophys J. 2008 Mar 1;94(5):1916–29.
- Giobbe GG, et al. Extracellular matrix hydrogel derived from decellularized tissues enables endodermal organoid culture. Nat Commun. 2019 Dec 11;10(1):5658.
- Willemse J, et al. Hydrogels derived from decellularized liver tissue support the growth and differentiation of cholangiocyte organoids. Biomaterials. 2022 May;284:121473.
- van Tienderen GS, et al. Modelling metastatic colonization of cholangiocarcinoma organoids in decellularized lung and lymph nodes. Front Oncol. 2023 Jan 18;12:1101901.
- van Tienderen GS, et al. Extracellular matrix drives tumor organoids toward desmoplastic matrix deposition and mesenchymal transition. Acta Biomater. 2023 Mar 1;158:115–131.
- Dahl-Jensen S, Grapin-Botton A. The physics of organoids: a biophysical approach to understanding organogenesis. Development. 2017 Mar 15;144(6):946–951.
- Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005 Oct;15(5):378–86.
- Heo JH, et al. Engineering the extracellular matrix for organoid culture. Int J Stem Cells. 2022 Feb 28;15(1):60–69.
- Tuning the elastic moduli of Corning® Matrigel® and collagen I 3D matrices by varying the protein concentration (CLS-AC-AN-449).
- Borries M, et al. Quantification of visco-elastic properties of a Matrigel for organoid development as a function of polymer concentration. Front Phys. 2020 Oct 8;8:579168.
- Kaur S, et al. Non-matrigel scaffolds for organoid cultures. Cancer Lett. 2021 Apr 28;504:58–66.
- Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014 Jul 18;345(6194):1247125.
- Kim J, et al. Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol. 2020 Oct;21(10):571–584.
- Ramadan R, et al. The extracellular matrix controls stem cell specification and crypt morphology in the developing and adult mouse gut. Biol Open. 2022 Dec 15;11(12):bio059544.
Measuring ECM stiffness
Optics11 Life offers a solution for assessing the ECM’s mechanical properties. This technology surpasses traditional methods that rely on indirect measures. It provides researchers with tools to measure the ECM’s mechanical properties, producing data that can serve as mechanical biomarkers for cellular behavior.
We have whitepapers detailing how researchers use Optics11 Life technology in ECM research. These documents emphasize the technology’s ability to measure the ECM’s stiffness accurately and its impact on organoids. Variations in ECM stiffness influence tissue and organ functionality. Furthermore, Optics11 Life technology applications extend to materials science and engineering, emphasizing the importance of understanding material mechanical properties.

