by Optics11 Life
© 2023 Optics11 Life B.V.
Revolutionizing wound care with the Pavone: mechanical forces as potential biomarkers in wound healing
Mechanical properties of wound sites significantly influence the healing rate and quality of newly formed tissue, making mechanics a potential label-free biomarker for tissue regeneration. Here, we introduce the Pavone – a high-throughput mechanical screening platform for thoroughly characterizing epidermal cells and supporting physiologically relevant in vitro models for wound healing.
Introduction
Injuries to the skin’s normal barrier function heal through a progressive cellular response involving fibroblasts, macrophages, endothelial cells, and keratinocytes to restore the skin’s integrity. During wound healing, mechanical forces are crucial to skin regeneration. Changes in tissue mechanical properties, such as stiffness and viscosity, affect cell behavior and wound healing quality1,2. Characterizing the mechanical properties of the skin, particularly at cell length scales, is becoming increasingly relevant in regenerative medicine, mainly because the mechanics of cells and their microenvironment regulate various biological processes involved in wound healing3.
However, conventional methods for mechanically characterizing tissues, such as an atomic force microscope (AFM), are complex and time-consuming. They often require sample preparations that compromise the tissue’s native mechanical properties. In this context, we introduce the Pavone as a powerful instrument to mechanically characterize cells, tissues, spheroids, organoids, and biomaterials in a non-destructive manner. This innovative technology can assay the mechanical properties of cells and their surroundings in health and disease conditions and elucidate the mechanical responses triggered by disturbances with the potential to inspire novel therapeutic strategies.
Optics11 Life novel technology
Pavone is a mechanical screening platform for novel research applications, such as disease modeling, drug screening and delivery, regenerative medicine, and tissue engineering. More specifically, it can monitor the mechanical environments in healthy, injured, repaired, and fibrotic tissues4. Moreover, it characterizes the influence of mechanical properties on biological processes linked to tissue healing, such as collective cell migration5,6 and wound contraction7. By applying this technology to developing human skin equivalents8 and organoids9, scientists can more accurately model the physical microenvironment of native tissues.
Besides, Pavone supports the development of scaffolds7,10 and drug delivery systems11. The mechanical properties of these materials are crucial to ensure compliance with injured tissue in vivo. This application note describes the mechanical properties of keratinocytes and their response to bioactive peptides using Pavone technology.
Mechanical characterization of keratinocytes
Pavone is a powerful tool for characterizing the mechanical features of epidermal cells in wound healing. Keratinocytes, the predominant cells in the epidermis, are responsible for the skin’s protective barrier function. Keratin cytoskeleton remodeling is essential for cell–cell and cell–matrix adhesion, modulating cell motility during wound healing12,13.
Here, we investigated the influence of two bioactive peptides on keratinocyte mechanical properties using Pavone. Bioactive peptides can influence the expression of cytoskeletal and cell–extracellular matrix–interacting proteins, which are crucial for tissue remodeling and wound healing13.
In this study, human keratinocyte cell lines (HaCaT) were treated with two distinct bioactive peptides (BP): BP1 (5 μg/ml) or BP2 (0.5 μg/ml). Untreated cells containing only culture medium were included as a control. After five days, HaCaT cells’ stiffness and topography were measured using the Pavone.
Cells’ mechanical properties were assessed using a probe with a stiffness of 0.2 N/m and a tip radius of 9.5 μm. The indentation depth was 3 μm. All indentations were performed in cell culture medium. To determine Young’s modulus and surface topography, the contact point was identified using Optics11 Life DataViewer software. A Hertzian model with a Poisson’s ratio of 0.5 was fitted to the load vs. indentation curve corresponding to the first 1000 nm of indentation. Any indentation with a fit for the Hertzian model below an R² value of 0.80 was excluded from further analysis.
Our findings revealed that exposure of HaCaT cells to BP1 and BP2 decreased cell stiffness, proportionally to the concentration of the investigated peptide (4.25 ± 1.08 kPa for cells treated with BP1, 3.18 ± 1.00 kPa for cells treated with BP2, and 5.50 ± 1.61 kPa for untreated cells) (Figure 1). Exposure to the peptides, particularly BP1, also influenced the cells’ morphology, which became rounded, unlike the typical flat appearance of untreated HaCaT. This might indicate cytoskeletal rearrangements that align with cell stiffness changes (data not shown).
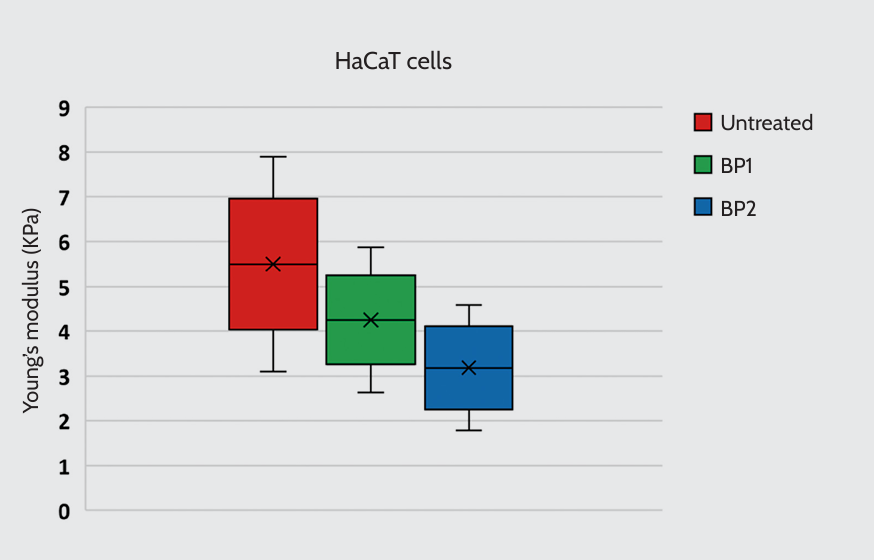
Young’s modulus of HaCaT cells. Cells were treated with two bioactive peptides (BP): BP1 (5 μg/ml) or BP2 (0.5 μg/ml). Untreated cells were included as a control.
Additionally, by mapping the surface of the untreated HaCaT cell monolayer, we could visually reconstruct the 3D topography of distinct cellular compartments, especially the cytoplasm (thicker area) and the cell–cell junctions (thinner area) (Figure 2).
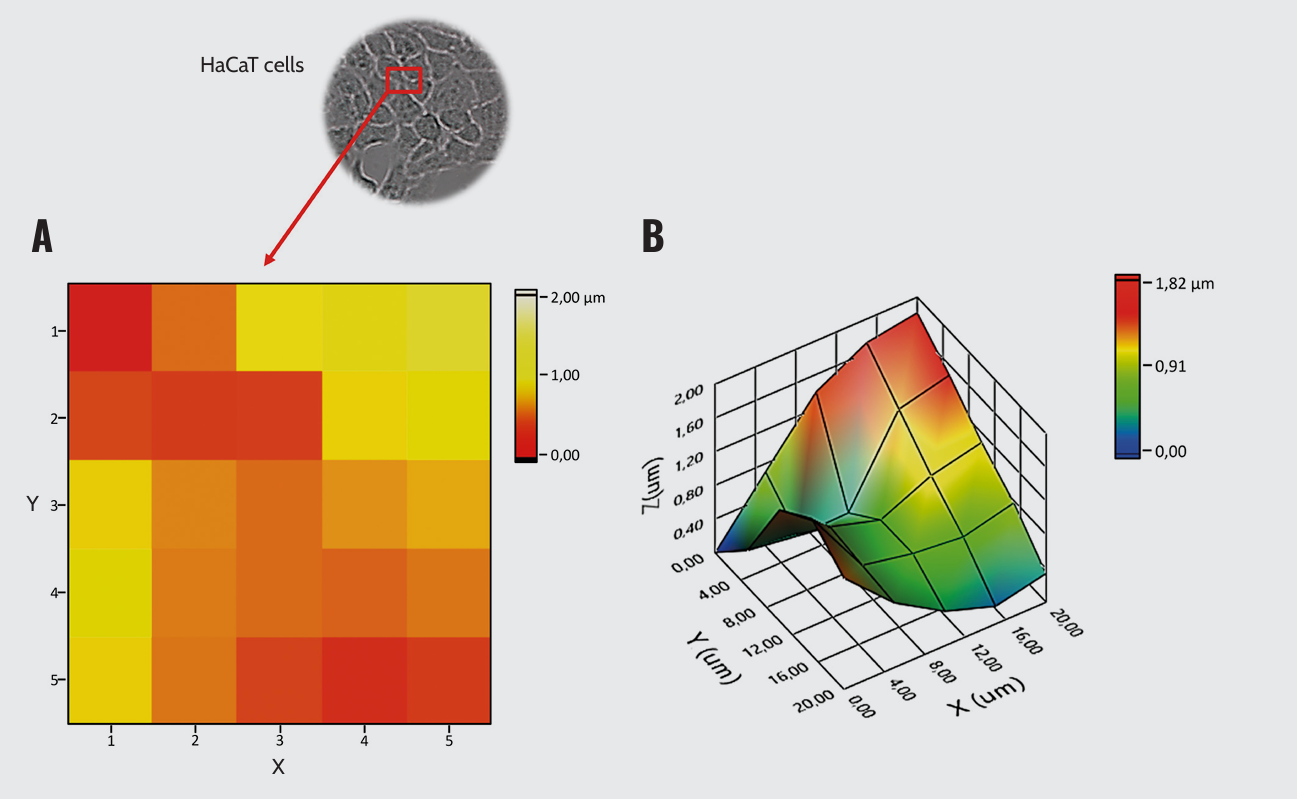
HaCaT cells topography. (A) 2D surface plot and (B) 3D surface topography.
Conclusion
- Pavone is a powerful tool to measure the mechanical properties of cells involved in wound healing, specifically keratinocytes.
- Pavone revealed how potential treatments influence cell stiffness, providing significant insights into the mechanics of keratinocytes with potential applications to skin regeneration and wound healing research.
The mechanical properties of wounded tissues affect the rate and quality of healing, making mechanics a potential label-free biomarker for tissue regeneration. In this context, Pavone can support novel wound care approaches by fostering a better understanding of tissue regeneration and healing and developing physiologically relevant in vitro models.
References
- Kobiela T, et al. The effect of anti-aging peptides on mechanical and biological properties of HaCaT keratinocytes. Int J Pept Res Ther. 2018;24(4):577–587.
- Mierke C. T. Viscoelasticity, like forces, plays a role in mechanotransduction. Front Cell Dev Biol. 2022 Feb 9;10:789841.
- Pensalfini M, Tepole A. B. Mechano-biological and biomechanical pathways in cutaneous wound healing. PLoS Comput Biol. 2023 Mar 9;19(3):e1010902. Cox T. R, Erler J. T. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech. 2011 Mar;4(2):165–78. doi: 10.1242/dmm.004077.
- Kimura S, Tsuji T. Mechanical and immunological regulation in wound healing and skin reconstruction. Int J Mol Sci. 2021 May 22;22(11):5474.
- Segars K. L, et al. Age dependent changes in corneal epithelial cell signaling. Front Cell Dev Biol. 2022 May 5;10:886721.
- Shellard A, Mayor R. Collective durotaxis along a self-generated stiffness gradient in vivo. Nature. 2021 Dec;600(7890):690–694. doi: 10.1038/s41586-021-04210-x. Epub 2021 Dec 8. Erratum in: Nature. 2022 Jan;601(7894):E33.
- Zhang H, et al. Purse-string contraction guides mechanical gradient–dictated heterogeneous migration of epithelial monolayer. Acta Biomater. 2023 Mar 15;159:38–48.
- Zhang H, et al. Purse-string contraction guides mechanical gradient–dictated heterogeneous migration of epithelial monolayer. Acta Biomater. 2023 Mar 15;159:38–48.
- Weigel T, et al. Fully synthetic 3D fibrous scaffolds for stromal tissues – replacement of animal-derived scaffold materials demonstrated by multilayered skin. Adv Mater. 2022 Mar;34(10):e2106780.
- Willemse J, et al. Hydrogels derived from decellularized liver tissue support the growth and differentiation of cholangiocyte organoids. Biomaterials. 2022 May;284:121473.
- Cao G, et al. Biomimetic SIS-based biocomposites with improved biodegradability, antibacterial activity and angiogenesis for abdominal wall repair. Mater Sci Eng C Mater Biol Appl. 2020 Apr;109:110538.
- Bono N, et al. Silk fibroin microgels as a platform for cell microencapsulation. J Mater Sci Mater Med. 2022 Dec 31;34(1):3.
- Walter M. N, et al. Mesenchymal stem cell-conditioned medium accelerates skin wound healing: an in vitro study of fibroblast and keratinocyte scratch assays. Exp Cell Res. 2010 Apr 15;316(7):1271–81.

Wounds, whether acute or chronic, involve a complex healing process that can be disrupted by various factors. This process can cause severe complications and significantly impact a patient’s quality of life. Therefore, understanding and tracking wound healing progression becomes paramount in wound care management.
Measuring Tissue Stiffness
In response to this challenge, our nanoindentors offer a unique solution. They empower researchers to measure the mechanical properties of tissues, providing valuable data that can serve as mechanical biomarkers for the healing process.
We provide application notes that delve into practical cases where researchers have utilized our nanoindenters in wound care research. Importantly, these notes highlight how these tools can accurately measure the stiffness of tissues and cells. This information is crucial because changes in tissue stiffness can indicate the progression or stagnation of the healing process. Furthermore, the applications of our nanoindenters extend beyond wound care research. They find use in a variety of other fields, including materials science and engineering, where understanding the mechanical properties of materials is essential.

