May 8 is recognized as World Ovarian Cancer Day, significantly raising awareness about this deadly disease, and celebrating continued research developments. Although the statistics concerning ovarian cancer are alarming, with over 200,000 deaths every year (Huang J. et al., 2022), there is hope due to recent advancements in the field.
The state of current research on ovarian cancer is showing promise, but there are still many challenges to overcome. The vague symptoms of this cancer often lead to late diagnoses, which in turn negatively impact survival rates (American Cancer Society, 2024). It is essential to continue researching the disease’s intricate mechanisms and developing early detection methods to improve outcomes.
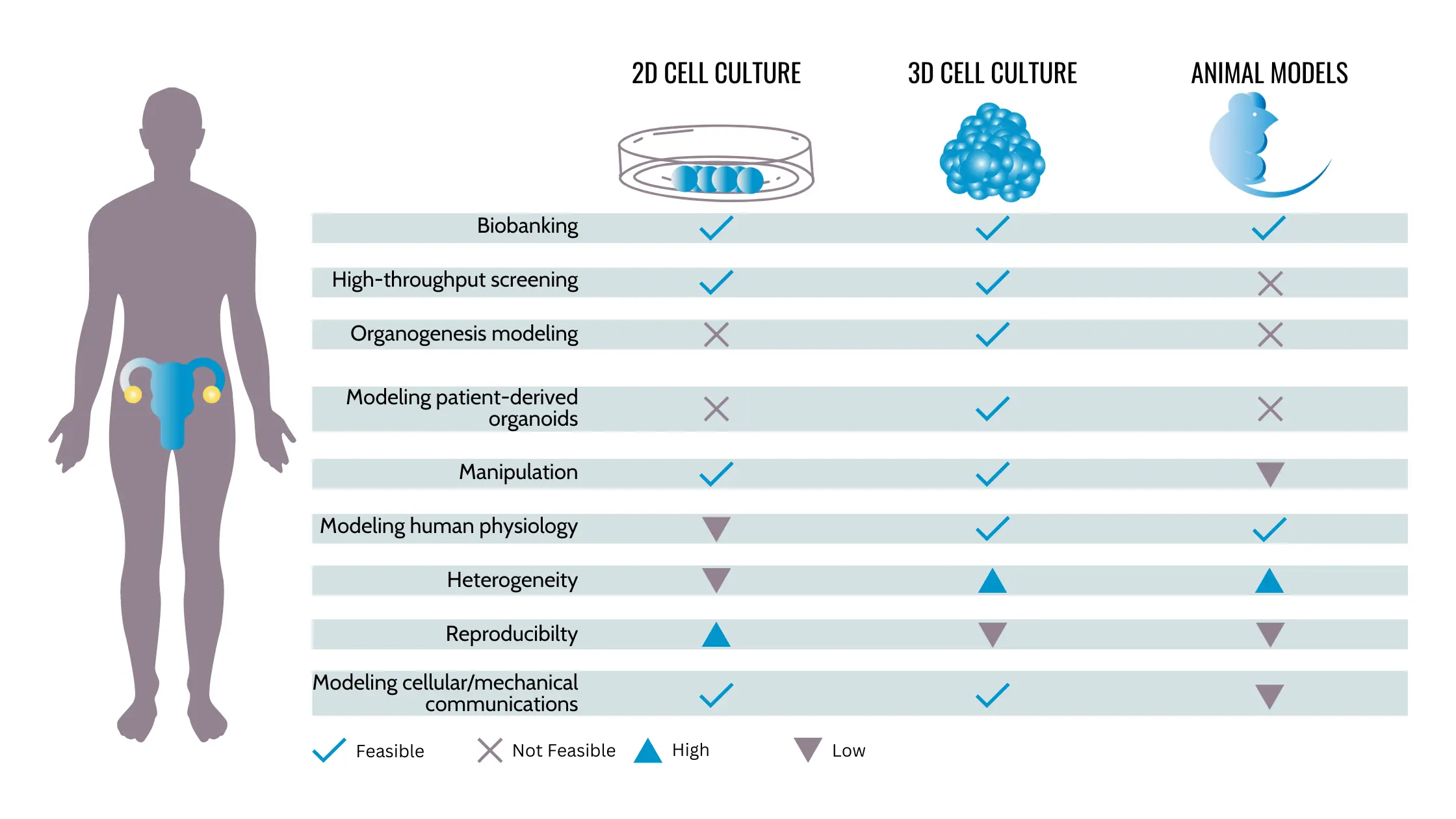
Cancer research has traditionally relied on 2D cell cultures, which have limited accuracy in replicating tumors. However, the emergence of 3D models, such as organoids, is changing the game. A study published by Clevers et al. (2020) highlights the potential of organoids, which are miniature 3D structures that mimic actual ovarian tissue. These can be used to study how specific mutations, such as Trp53 and Brca1, contribute to the development of ovarian cancer.
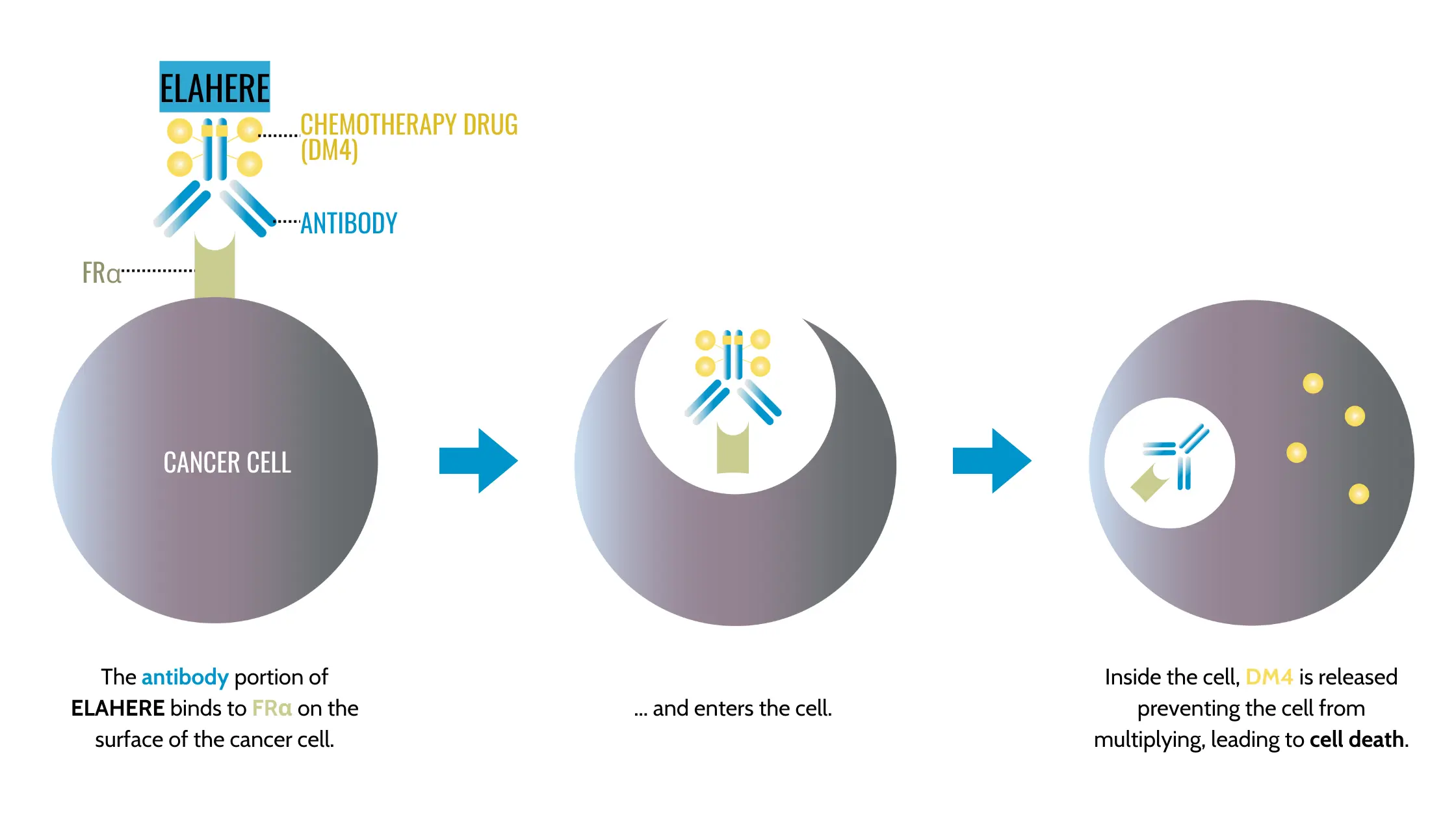
Exciting new treatments are being developed for cancer patients beyond traditional methods. Recently, the FDA approved drugs such as Elahere (mirvetuximab soravtansine-gynx) (U.S. Food and Drug Administration, 2022 & 2024) for platinum-resistant ovarian cancer. These drugs target specific vulnerabilities in cancer cells, providing much-needed hope for patients who have exhausted the more traditional treatment options (learn more about these advancements at U.S. Food and Drug Administration, 2024 & Farrell, 2024).
Today, on World Ovarian Cancer Day, we rise together. Not just in awareness, but in action. By bolstering early detection efforts through heightened awareness and research investment, we can significantly improve patient outcomes.
References
American Cancer Society. (2024). Survival rates for ovarian cancer. https://www.cancer.org/cancer/types/ovarian-cancer/about/key-statistics.html
Huang, J., Chan, W.C., Ngai, C.H., et al. Worldwide Burden, Risk Factors, and Temporal Trends of Ovarian Cancer: A Global Study. Cancers (Basel). 2022;14(9):2230. Published 2022 Apr 29. doi:10.3390/cancers14092230
Clevers, H., et al. (2020). Assessing the origin of high-grade serous ovarian cancer using CRISPR-modification of mouse organoids. Utrecht University. https://www.uu.nl/en/publication/studying-the-development-of-ovarian-cancer-with-organoids
Heydari, Z., Moeinvaziri, F., Agarwal, T. et al. Organoids: a novel modality in disease modeling. Bio-des. Manuf. 4, 689–716 (2021). https://doi.org/10.1007/s42242-021-00150-7
Farrell, J. (2024, March 22). AbbVie’s new biological missile ovarian cancer treatment gets full FDA approval. Forbes. https://www.forbes.com/sites/jamesfarrell/2024/03/22/abbvies-new-biological-missile-ovarian-cancer-treatment-gets-full-fda-approval/
U.S. Food and Drug Administration. (2022, November 14). FDA grants accelerated approval for mirvetuximab soravtansine-gynx (mirvetuximab) for the treatment of HRD-positive, platinum-resistant ovarian cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-mirvetuximab-soravtansine-gynx-fra-positive-platinum-resistant
U.S. Food and Drug Administration. (2024, March 22). FDA approves elahere for platinum-resistant ovarian cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-mirvetuximab-soravtansine-gynx-fra-positive-platinum-resistant-epithelial-ovarian
World Ovarian Cancer Coalition. (2020). World ovarian cancer atlas. https://worldovariancancercoalition.org/wp-content/uploads/2020/10/2020-World-Ovarian-Cancer-Atlas_FINAL.pdf



